Events
Include expired: true
and City: Bogota or Athens or Berlin or Oeiras
and Target audience: software developers, bioinformaticians or Pharmaceutical
TeSS makes use of some necessary cookies to provide its core functionality. Additionally, we make use of Google Analytics to discover how people are using TeSS in order to help us improve the service. To opt out of this, choose the "Allow necessary cookies" option.
See our Privacy Policy for more information.
You can modify your cookie preferences at any time here, or from the link in the footer.
Include expired: true
and City: Bogota or Athens or Berlin or Oeiras
and Target audience: software developers, bioinformaticians or Pharmaceutical
In the left-hand column of the Google Calendar main view, click the arrow to the right of "Other calendars" and click "Add by URL". In the form that appears, paste in the URL from the box above, and click the button to confirm.
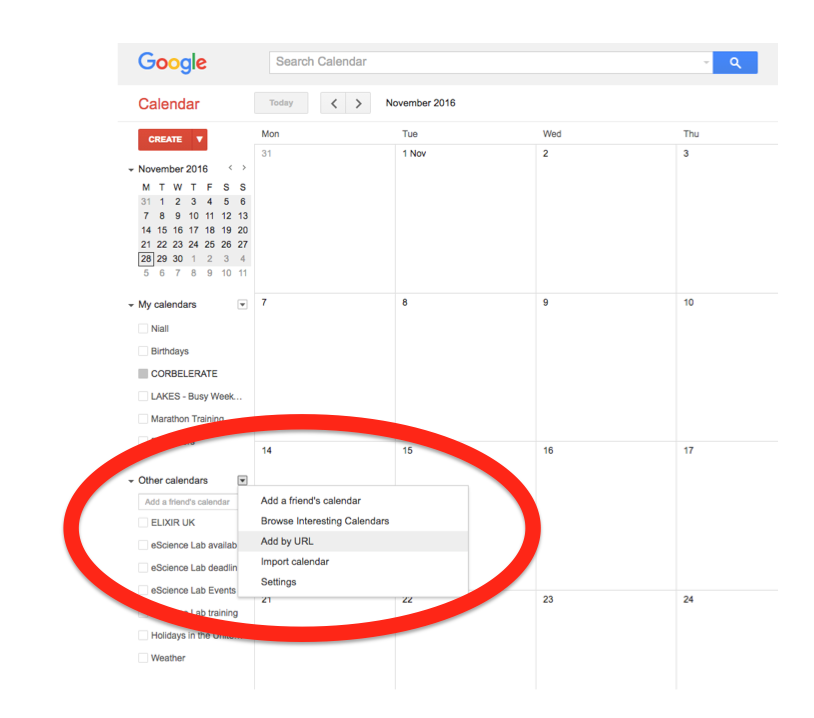
Please note, it may take a while for newly created events in TeSS to synchronise with your Google Calendar.
27 - 29 September 2017
Berlin, Germany
Face-to-face
R&D Support Medical Writing Submissions QA/QC6 May 2019 @ 14:30 - 18:00
Oeiras, Portugal
Face-to-face
Biodata, Bioinformatics, Biodata
Note, this map only displays events that have geolocation information in
TeSS.
For the complete list of events in TeSS, click the grid tab.